Background: Ide-cel is a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T cell (CART) therapy approved for RRMM after ≥4 prior lines of therapy (LOT), based on the results of the pivotal KarMMa trial. While response rates and survival outcomes have been very promising, patients (pts) with significant comorbidities, poor performance status (PS), aggressive disease characteristics, and prior BCMA-directed therapy (BDT) exposure were excluded from the KarMMa trial. There is an unmet need, therefore, to characterize outcomes in this real-world population. In this multicenter study, we evaluated real-world outcomes of pts not meeting the KarMMa trial eligibility criteria, treated with standard of care (SOC) ide-cel.
Methods: Three US academic centers, part of the US Myeloma Innovations Research Collaborative, contributed data to this retrospective analysis, which included pts who had undergone apheresis up until 5/15/2023 and who were infused with SOC ide-cel. We compared our cohort (SOC cohort) with the KarMMa cohort for safety outcomes, including cytokine-release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), infections, and hematologic toxicities, as well as efficacy outcomes, including overall response rate (ORR), complete response or better (≥CR), progression-free (PFS) and overall survival (OS). Binomial exact test and Kaplan Meier method were used for statistical analysis.
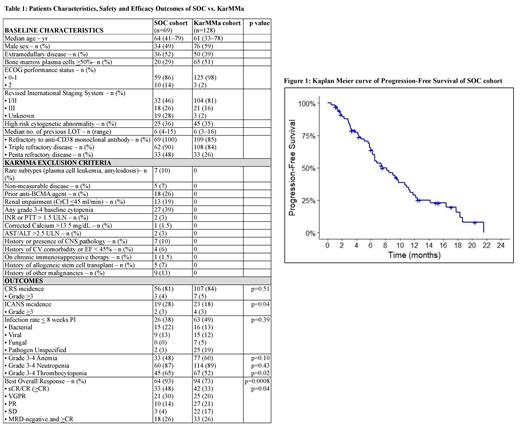
Results: 120 pts were leukapheresed, 77 (64%) did not meet the KarMMa eligibility criteria, and 69 (58%) of them proceeded with ide-cel infusion and had at least 30-day follow-up available for safety and efficacy assessments. Among the 69/120 SOC ide-cel recipients included in the analysis, median age was 64 (range 41-79) years and 49% were male. The main reasons for not meeting KarMMa inclusion criteria were: baseline 3-4 cytopenia (39%), prior BDT (26%), renal impairment (CrCl <45 ml/min) (19%), ECOG PS ≥2 (14%) and history of other malignancy (13%); the rest are summarized in Table 1.Pts were heavily pretreated with a median of 6 (4-18) prior LOT and 36% had high-risk cytogenetics (del-17p, t[4;14], t[14;16], t[14;20]) prior to ide-cel infusion. The SOC cohort had more extramedullary disease (52% vs 39%, p=0.02), whereas the KarMMa cohort had higher median bone marrow plasma cell percentage (51% vs 29%, p=0.0002); other baseline characteristics including age, gender, prior LOT, high-risk cytogenetics, and prior refractoriness were similar among the two cohorts. CRS occurred in 81% (grade ≥3, 4%) of SOC pts vs 84% (grade ≥3, 5%) of KarMMa pts (p=0.51). ICANS occurred in 28% (grade ≥3, 3%) of SOC pts vs 18% (grade ≥3, 3%) of KarMMa pts (p=0.04). Infection rate < 8 weeks post-infusion for SOC vs KarMMa cohorts was 38% vs 49% (p=0.39) (bacterial 22% vs 13%, viral 13% vs 12%, fungal 0% vs 5%). Grade 3-4 cytopenias post-infusion for SOC vs KarMMa cohorts were as follows: anemia 48% vs 60% (p=0.10), neutropenia 87% vs 89% (p=0.43), and thrombocytopenia 65% vs 52% (p=0.02). With a median follow-up of 10 and 13.3 months (mo) for SOC and KarMMa cohorts, respectively, ORR was paradoxically higher for the SOC cohort (93% vs 73%, p=0.0008); likewise, ≥CR rate was also higher for the SOC cohort (48% vs 33%, p=0.04). The median PFS of SOC pts (7.6 mo, 95% CI, 6.2-10.9) (Figure 1) was comparable to KarMMa pts (8.8 mo, 95% CI, 5.6-11.6) (c-index 0.96). Likewise, median PFS of SOC pts who achieved ≥CR (10.9 mo, 95% CI, 9-18.3) was comparable to KarMMa pts (20.2 mo, 95% CI, 12.3-NR) (c-index 0.75). The median OS of SOC vs KarMMa cohorts were similar 19.4 mo (95% CI, 12.5-NR) vs 19.4 mo (95% CI, 18.2-NR) (c-index 0.7).
Conclusion: Most safety aspects including CRS, infections and hematological toxicities (except thrombocytopenia) were similar between our real-world cohort and the pts who received ide-cel in the KarMMa study. ICANS was higher in the SOC cohort, however, rate remained low, and most events were grade 1-2. The survival outcomes were comparable between the two cohorts, with responses being, interestingly, higher in the SOC cohort. Our results demonstrate that ide-cel has comparable efficacy and safety in the real-world population, including those with prior BCMA therapy exposure, grade 3-4 cytopenias, ECOG ≥2 and significant comorbidities. These findings support broadening the inclusion criteria of future trials evaluating ide-cel in an effort to improve accessibility across RRMM pts.
Disclosures
Khouri:GPCR Therapeutics: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events. Hashmi:Karyopharm: Speakers Bureau; BMS: Honoraria; Jannsen: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau. McGuirk:Novartis: Research Funding; EcoR1 Capital: Consultancy; Allovir: Consultancy, Research Funding; Magenta Therapeutics: Consultancy; Fresenius Biotech: Research Funding; Astellas Pharma: Research Funding; Juno Therapeutics: Consultancy; Kite: Consultancy, Research Funding; Bellicum Pharmaceuticals: Research Funding; Gamida Cell: Research Funding; Pluristem Therapeutics: Research Funding. Ahmed:Kite, a Gilead company: Research Funding; Bristol Myers Squibb: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal